A study completed in August 2021 at the Georgia Institute of Technology has determined that heat-controlled Chimeric Antigen Receptor (CAR) T-cells may be able to predict cancer relapse. Chimeric Antigen Receptor T-cells are immune system cells (T-cells) charged in a laboratory to attack cancer cells. An antigen is any substance that causes the immune system to produce antibodies. Antibodies are crucial to fighting disease because they are blood proteins that latch onto antigens and remove them from the body.
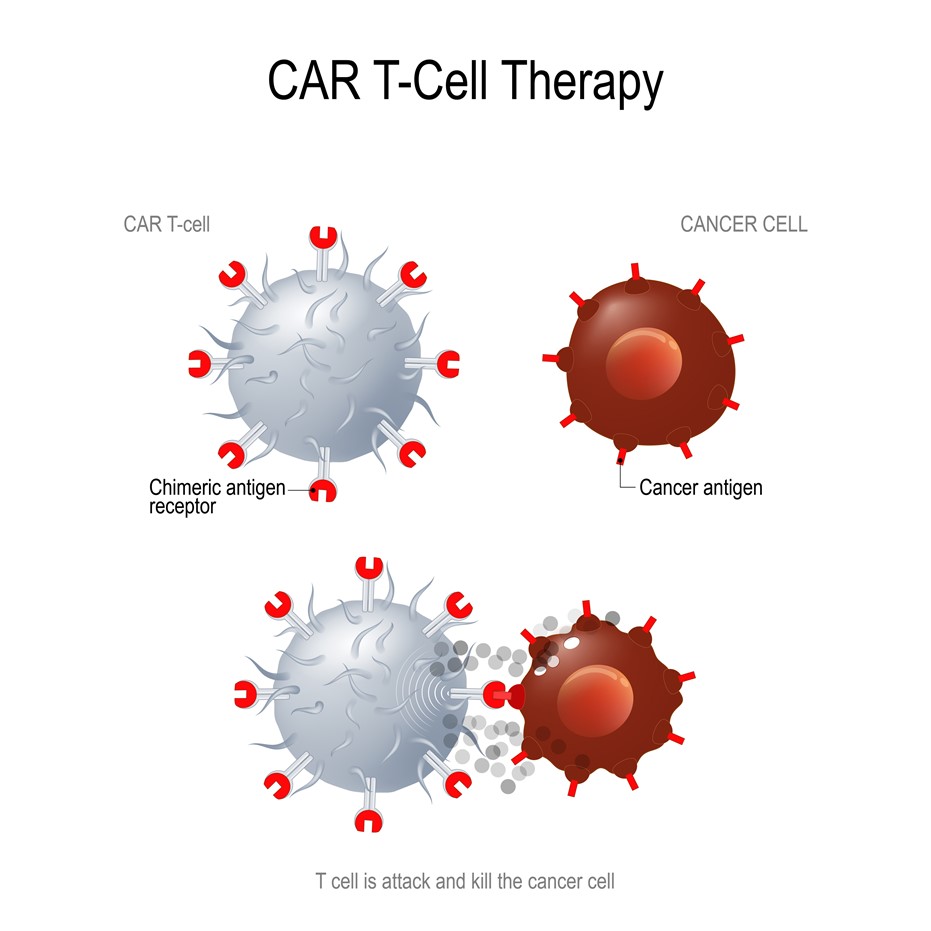
CAR T-cell therapy is an advanced version of adoptive cell transfer. Adoptive cell transfer is how patients’ immune cells are collected and used to treat their cancer. The Food and Drug Administration (FDA) initially approved CAR T-cell therapy in 2017 to treat children who have acute lymphoblastic leukemia.
The foundation of CAR T-cell therapy is T-cells, commonly referred to as the workhorses of the immune system. This therapy requires that doctors draw blood from patients, separate T-cells and then genetically engineer the cells to produce receptors on their surface called Chimeric Antigen Receptors.
CAR T-Cell Therapy Development
The development of this technology has been possible through a variety of research tools and through centrifuges which draw out T-cells from potential patients. We are proud to supply devices that help researchers study, diagnose and treat cancer. C & A has been a leader in improving the health and minds of people worldwide for the past thirty years, and we are excited to supply researchers with tools that will allow them to develop this therapy further.
Since the introduction of this therapy, researchers have been investigating whether it can impact solid cancers, like colorectal and breast tumors. Steven Rosenberg of the National Cancer Institute’s Center for Cancer Research has been the first to report successful cancer treatment with CAR-T cells.
Continued Research to Improve T-Cells
Since 2017, advances in intracellular engineering have improved the ability of these T-cells to produce more of themselves after infusion and survive longer in the blood’s circulation. Intracellular engineering is how methods of engineering are applied to the inner working of cells. When replicated or edited in a laboratory, doctors can alter cells at a fundamental level through biological interference. Additionally, it would have taken several weeks for labs to create a bath of edited T-cells in the past. However, this can now be completed in a week. While this therapy has been primarily focused on pediatric trials, trial groups for adults are currently being conducted.
Potential Risks
While this therapy will improve blood cancer treatment, it can also cause a potentially deadly side effect called cytokine release syndrome. Cytokines are molecules that can signal and aid cell to cell communication for immune system response. Typically, cytokines play a valuable role in preparing the body to fight off illness. However, if the body identifies a threat but releases too many cytokines to fight it off, these cytokines can cause a significant amount of harm to internal organs. Cytokine release syndrome occurs when cytokines are released in the blood, leading to extremely high fevers and dangerous drops in blood pressure. There are also other potential adverse side effects, including B-cell aplasia and cerebral edema.
Whether you are working in cancer research or educating tomorrow’s researchers, C & A has a line of reliable centrifuges that can meet any of your laboratory’s testing needs. For more information, please check out our Histology Catalog.
C & A Scientific is a dedicated leader in improving the health and minds of people worldwide. We supply over 700 award-winning medical and STEM-inspired products to distributors and retailers looking for sensational customer service. Learn more about us and our story here.

